|
Hello,
I hope you're doing well and will
find the attached articles both interesting and useful. The first is about how heat affects fungi by Dr.
Harriet Burge and the second is about the fungi Bipolaris by Dr. Michelle Seidl.
With best wishes,
Dave Gallup
How Does Heat Affect
Fungi?
By Dr. Harriet
Burge
The effects of heat on fungi
depend on many factors, including the genus, species and strain of the fungus, the amount of available water,
kinds of nutrients, and many other environmental factors. Of course, temperature is also a crucial factor.
Most of the research on temperature relationships for the fungi has been done in the food industry where heat
is commonly used to prevent fungal growth. Much of this research involves wet heat, which is more effective
than dry heat.
Fungi can be divided into groups
according to temperature requirements for optimal growth. Note that these requirements are dependent on water
availability and nutrients, and are measured under carefully controlled conditions. These same terms are used
for bacteria, but the temperature ranges differ.
The groups are as follows:
-
Psychrophiles: with optima less
than 10°C
-
Mesophiles: with optima in the
room temperature range (18-22°C)
-
Thermophiles: with optima at or
above 37°C
There are also categories
defining the ability of the fungi to withstand different temperature regimes. In this case, we have
psychrotolerant and thermotolerant fungi, indicating that growth can occur at either low or high
temperatures, but is not optimal. Thus, some mesophilic fungi may be able to grow or at least survive at
either low or high temperatures, depending on the genetics of the strain and other environmental conditions.
Another form of temperature tolerance lies in the spores, which can often withstand temperature extremes, and
germinate when conditions return to normal. Finally, some fungi that are normally mesophilic have spores that
require heat to stimulate germination. Temperature tolerance is strongly tied to the amount of water so that
wet heat is much for effective at damaging spores than dry.
The effects of heat on fungi are
related to the chemical reactions within the fungal cells. For optimum growth, temperatures must be in a
range that allows the most efficient progression of the chemical reactions necessary for growth. As
temperatures progress above the optimum temperature, the chemical reactions occur less efficiently, and
growth slows. Eventually, the temperature can reach a point where growth stops, and cell components begin to
be actually damaged by the heat. Enzymes are proteins that change structurally when heated to their limit of
tolerance. Likewise, membranes, which contain lipids, change in structure, and their function of protecting
and regulating the internal environment of the cell becomes compromised.
Most fungi are mesophilic, and
have growth optima within the temperature range that people find comfortable. This is why so many fungi
appear when moisture enters our homes, schools, and work environments. Because of air conditioning and
heating, mesophilic fungi flourish in occupied environments in all climates. However, the fungal species that
are abundant outdoors may vary considerably from one climate to another. In hot dry climates, fewer species
of fungi are present, both because of the lack of water and the high temperatures. Thus, thermophilic and
xerophilic (dry tolerant) fungi are likely to be more abundant than in cooler wetter environments. In
tropical and subtropical places where both heat and moisture are present, thermophilic and thermotolerant
fungi with mesophilic water requirements tend to be abundant. The incidence of fungal infections (including
sinus infections) tends to be higher in these areas in part because the fungi that can withstand human body
temperatures are more abundant than in temperate climates. Finally, continental climates that tend to swing
from hot humid to cold dry conditions have a few overwhelmingly dominant fungi (e.g., Cladosporium
species) with other mostly mesophilic fungi filling in the gaps. Of course, this is an over-simplification,
especially since, as mentioned many times, an array of factors are necessary for optimal growth of all kinds
of fungi. Also, fungi live in microenvironments that may have very different temperature/water conditions
than are represented by climate.
Table 1 lists some of the fungal
temperature relationships that have been reported in the literature.
Table 1:
|
Activity
|
Temperature and Duration
|
Notes
|
|
Hot dry air
sterilization
|
170 °C (340 °F), 1
hour
160 °C (320 °F), 2 hours
150 °C (300 °F), 2.5 hours
140 °C (285 °F), 3 hours
|
Kills virtually all
spores
|
|
Wood heat treatment for rot
resistance
|
200 °C (392 °F), 24
hours
|
Changes structure and color
of the wood
|
|
Whole house treatment for
termites and/or fungi
|
71°C (160 °F), 4-6
hours
|
|
|
Penicillium spore
death in water
|
54.4 °C (130 °F), 30
minutes
|
|
|
Ascospores activation in
grape juice
|
70 °C (158 °F), 30
minutes
|
This is higher than the
temperature which kills Penicillium in water
|
|
Germination of chlamydospores
in Cladosporium-related species stimulated by moist heat
|
75 °C (167 °F), 30
minutes
|
This is higher than the
temperature which kills Penicillium in water
|
The question sometimes arises
about heating an entire structure as part of remediation. In this process, an entire house (or part of a
building) may be heated to a temperature that should kill most of the spores remaining following structural
remediation and any invisible growth. Clearly, using heat will help the building dry faster because it
reduces the relative humidity and increases the vapor pressure of the water in the building. Heat may also
damage some plastics, some types of electrical equipment, and other items; so, naturally, heat sensitive
items will need to be removed prior to heating the structure. There may also be spores that are particularly
resistant to heat, microclimates that do not attain high enough temperatures, or spores that are stimulated
to germinate. Also, spores that are left may still remain allergenic. As we do not have expertise in this
method of remediation, and the science in this area is still in its infancy, we do not feel qualified to
provide “the answer†for if or when heat remediation is good or bad. Also,
like many aspects of fungal investigations it depends upon many situation specific criteria. Finally, it is
important to remember that spores are continually entering the structure and the only permanent fix for
fungal contamination is the maintenance of dry conditions throughout any building.
Fungus of the Month:
Bipolaris
By Dr. Michelle
Seidl
Bipolaris is relatively
common with approximately 75 described species occurring worldwide. As described by Shoemaker (1959),
Bipolaris is a filamentous, dematiaceous (darkly pigmented), anamorphic (asexual, conidial or
mitosporic) fungus possessing phragmospores (multicellular spores). This anamorphic genus is well correlated
with the teleomorphic (sexual or meiosporic) genus Cochliobolus in the Pleosporaceae family of
Ascomycetes.
Bipolaris is considered
cosmopolitan, associated primarily with grasses, but also occurs on a variety of plant materials, in soil,
decaying food, and indoors on various substrates (Knudsen et al., 1995). A few species, e.g. B.
australiensis, B. hawaiiensis and B. spicifera, are well-documented human pathogens.
Spores from Bipolaris,
Dreschlera and Exserohilum are not differentiated on spore trap and usually not on direct exam
samples unless there is growth and the sporulating structures are recovered. Difficulty in differentiation is
due to similarities in appearance of the spores and the critical features necessary to accurately identify
the genera are usually lacking. Some laboratories also group Helminthosporium in with the previous
three, however, Helminthosporium can be distinguished by its obclavate (larger at the base) spore
shape and straight conidiophores.
In the outside air, spores in the
Bipolaris/Drechslera group are occasionally recovered in spore trap samples. They are recovered most
frequently during the fall season. Recovery rates vary from about 10% in February to 23% in September. When
recovered, the spore density is relatively low, with the 50th percentile value being about 13 spores per
cubic meter, which is marginally above the detection limit for many samples.
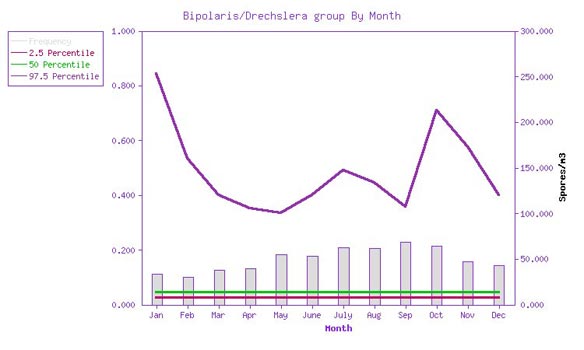
Figure 1: Bipolaris/Drechslera
group frequency of detection and spore density by month.The gray bars represent the frequency of
detection, from 0 to 1 (10%), graphed against the left axis. The red, green, and purple lines represent the
2.5, 50, and 97.5 percentile airborne spore densities, when recovered, graphed against the right hand axis.
(Source: EMLab™ MoldRange data. Total sample size for this graph: 39,878.)
Recovery rates and spore levels
are still low even in various weather conditions. In this dimension, recovery rates vary from about 6% in
moderate rain to 18% in clear weather. When recovered, the spore density is again low, with the 50th
percentile value being about 13 spores per cubic meter. Again, this is marginally above the detection limit
for many samples.
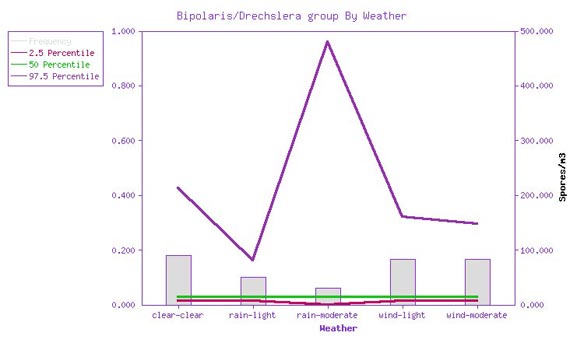
Figure 2: Bipolaris/Drechslera
group frequency of detection and spore density vs. weather. The gray bars represent the frequency of
detection, from 0 to 1 (10%), graphed against the left axis. The red, green, and purple lines represent the
2.5, 50, and 97.5 percentile airborne spore densities, when recovered, graphed against the right hand axis.
(Source: EMLab™ MoldRange data. Total sample size for this graph: 20,191.)
With culturable samples,
differentiation between these genera and some species level identification is possible. Dematiaceous fungi
are conidial fungi that produce dark brown, black or green colonies. Colonies of Bipolaris are
initially whitish (prior to spore maturation) becoming grey to dark olive to black on the surface. The
colonies of Bipolaris are dark on the reverse, both initially and when mature. The growth rate is
rapid and it has tolerance to the fungicide benomyl (Hoog et al., 2000). Colony texture is suede-like to
hairy or downy. Microscopically, the conidia (asexual spores) are brown, ellipsoidal to fusoid, straight or
slightly curved, typically ranging in size from 4-17 microns wide and often up to 120 microns long, with one
species up to 250 microns. They are multicellular, appearing thick-walled and with the central cells not much
darker and broader than the end cells. Conidia are produced along a main axis which appears bent or as a
zigzag.
The key feature of
Bipolaris spores is the germination pattern in that they grow and develop only at the ends
(bipolar). These spores are unique in that they are not made up of normal cells separated by septae
(cross-walls). Instead the cells are compartmentalized by distosepta, meaning they are contained in sacs that
have a wall distinct from the outer wall of the conidium. This feature cannot be accurately determined using
the light microscope alone and requires higher magnification than is used for analyzing spore trap samples.
After spore germination, the hyphae of Bipolaris are septate and brown.
In the indoor environment,
Bipolaris that has been recovered from the air is generally from outdoor sources, however it has
been occasionally recovered growing on indoor substrates such as wood, plant materials, and some food
stuffs.
Historically, Bipolaris
has been described under Dreschlera. Despite the similarities between genera, species of
Dreschlera have straight conidia (spores) that germinate from any cell and develop perpendicular to
the main axis. Bipolaris germinates only from the end cells and develops along the main axis.
Exserohilum is distinct from the other two genera by possessing a protruding or prominent attachment
point. Both Bipolaris and Dreschlera lack this feature. There are two additional genera
that may be grouped here as well. Curvularia is somewhat similar but differs by a few features
including having truly septate conidia that are usually slightly curved. As mentioned above,
Helminthosporium spores are straight and larger at the base. Due to morphological similarities,
confusion has resulted in the nomenclature between these five genera. For example, Bipolaris and
Exserohilum were once grouped under the name Helminthosporium. According to St-Germain and
Summerbell (1996), Helminthosporium is limited to plants. Clinical cases reported in the past as
caused by Helminthosporium are now identified as Bipolaris or Exserohilum. As a
plant pathogen, Helminthosporium causes various turf grass diseases such as leaf spot, root rot and
crown rot. Bipolaris causes leaf spot and melting out of tall fescue grass. It favors high
temperatures and normally develops in late summer. In addition, species of Dreschlera,
Curvularia and Exserohilum all occur on grasses (Sivanesan 1987). Dreschlera leaf
spot is similar in appearance but is more common in late spring to mid-summer, favoring long periods of leaf
wetness and moderate temperatures as this disease is inhibited by hot weather (Maloy 2005).

Bipolaris is
occasionally found in humans and other mammals. In older literature, it is a causative agent for the disease
(primarily in cattle) referred to as ‘helminthosporiosis’. In humans,
Bipolaris mainly causes allergic sinusitis and is also linked to eye infections (keratitis),
allergic bronchopulmonary disease, abdominal cavity inflammation (peritonitis), hay fever, asthma and other
respiratory problems (Washburn et al., 1988). These conditions are generally treatable.
Bipolaris is a causative
agent of phaeohyphomycosis, which is a fungal infection characterized by dematiaceous septate hyphae,
yeast-like growth, or a combination of both (McGinnis et al., 1986). Colonization may become chronic
or invasive in the case of impaired immunity. If the fungus becomes invasive, bone erosion may occur which
can also lead to brain lesions (Killingsworth and Wetmore 1990). Adam et al. (1986) reported
Bipolaris and Exserohilum infections, demonstrating the capability of both fungal genera to
cause invasive as well as "allergic" disease. They note that possibly all cases of Helminthosporium
and Dreschlera infections reported in the literature are caused by Bipolaris or
Exserohilum, indicating infections due to the latter two genera are more common than previously
recognized.
In the immunocompromised
individual, Bipolaris and Exserohilum are known to cause tissue death, central nervous
system disease, and vascular invasion and osteomyelitis (inflammation of bone and bone marrow). Sinusitis,
the most common form of disease caused by Bipolaris and Exserohilum, occurs in otherwise
healthy patients as nasal polyp formation and allergic rhinitis (allergic or non-allergic inflammation and
irritation of the nose). In summary, the diseases that have been documented specifically for
Bipolaris include: allergic sinusitis, subcutaneous infections, endocarditis (inflammation of the
heart membrane), pulmonary disease, cerebral disease and meningoencephalitis. The most common
Bipolaris species indicated in human disease is Bipolaris spicifera.
In closing, this large fungal
genus is primarily associated with plants and occasionally found in the indoor environment. Cases affecting
humans do exist, becoming serious in individuals with impaired immune systems.
References:
Adam, R. D., E. A. Petersen, M. A. Sabolle, M. G. Rinaldi, J. G. Corcoran, J. N. Galgiani and R. E. Sobonya.
1986. Phaeohyphymycosis caused by the fungal genera Bipolaris and Exserohilum. A report of 9
cases and review of the literature. Medicine 65(4):203-217.
Alcorn, J. L. 1983. Generic concepts in Dreschlera , Bipolaris and Exserohilum.
Mycotaxon 17:1-86.
Alexopolous, C.J., C. W. Mims & M. Blackwell. 1996. Introductory Mycology – 4th ed.
John Wiley & Sons, Inc.
Berbee, M. L., M. Pirseyedi and S. Hubbard. 1999. Cochliobolus phylogenetics and the origin of known,
highly virulent pathogens, inferred from ITS and glyceraldehydes-3-phosphate dehydrogenase gene sequences.
Mycologia 91:964.
Hoog, G. S. de, J. Guarro, J. Gene & M. J. Figueras. 2000. Atlas of Clinical Fungi –
2nd ed. Utrecht: Centraalbureau voor Schimmelcultures.
Killingsworth, S. M. and S. J. Wetmore. 1990. Curvularia/Dreschlera sinusitis. Laryngoscope
100:932-937.
Knudsen, I. M. B., J. Hockenhull and D. F. Jensen. 1995. Plant Pathology 44(3):467-477.
Maloy, O. C. 2005. Plant Disease Management. The Plant Health Instructor. DOI:
10.1094/PHI-I-2005-0202-01.
McGinnis, M.R., M.G. Rinaldi and R.E. Winn. 1986. Emerging agents of phaeohyphomycosis: pathogenic species
of Bipolaris and Exserohilum. J. Clin. Microbiol. 24:250-259.
Sivanesan, A. 1987. Graminicolous species of Bipolaris, Curvularia, Dreschlera, Exserohilum and their
teleomorphs. Wallingford, Oxon: C.A.B. International.
St-Germain, Guy and R. Summerbell. 1996. Identifying Filamentous Fungi – A Clinical
Laboratory Handbook, 1st ed. Belmont, CA: Star Publishing Company.
Washburn, R. G., D. W. Kennedy, M. G. Bagley, D. K. Henderson & J. E. Bennett. 1988. Chronic fungal
sinusitis in apparently normal hosts. Medicine 67:231-247.
The data and other information
contained in this newsletter are provided for informational purposes only and should not be relied upon for
any other purpose. Environmental Microbiology Laboratory, Inc. hereby disclaims any liability for any and all
direct, indirect, punitive, incidental, special or consequential damages arising out of the use or
interpretation of the data or other information contained in, or any actions taken or omitted in reliance
upon, this newsletter.
|

